3D printing of drugs
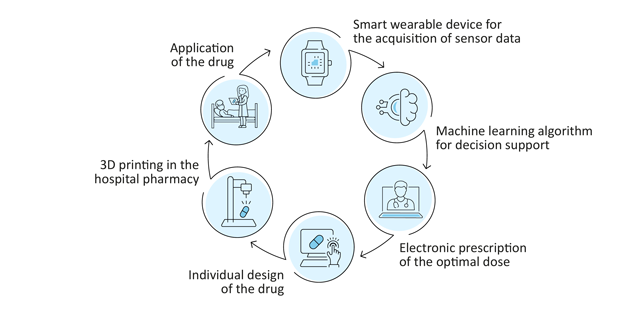
In a feasibility study, scientists from the hospital pharmacy of the University Medical Center Hamburg-Eppendorf (UKE) aim to demonstrate that 3D printing of drugs can in principle be integrated into the existing digital medication process at the UKE. A machine learning algorithm is to be developed to determine the optimal dose for each individual patient using data from smart wearable devices. The European Regional Development Fund (ERDF) of the European Union (EU) with around 650,000 euros funds the project „Patient-specific 2D/3D printing of drugs in Closed Loop Medication Management“until the beginning of 2023.
In a first step, the researchers identified and procured a suitable 3D printer with which drugs can be produced according to pharmaceutical quality criteria. This 3D printer can be used to manufacture patient-specific doses of drugs from filament, powder mixtures and semi-solid preparations. They also identified a suitable and clinically relevant active ingredient. To do this, they surveyed physicians from various disciplines and pharmacists in a UKE-wide online survey and in interviews. An interdisciplinary panel of experts then defined evaluation criteria, taking into account galenic, clinical and machine learning aspects.
Development of a formulation
In a next step, the scientists want to develop a suitable formulation with which the drug can be manufactured with the 3D printer according to pharmaceutical quality criteria in different dosages. The printed drug will then be evaluated for compliance with quality criteria according to the European Pharmacopoeia. This task is carried out by a team of pharmacists with experience and expertise in the field of formulation development and analytics of pharmaceutical products. The project does not include a clinical trial and the printed drugs will exclusively be tested in laboratories.
Collaboration with the Institute for Applied Medical Informatics
In addition, the researchers intend to use data obtained from smart wearable devices and evaluate them with the help of a machine learning algorithm in order to simulate the adjustment of dosages and thus further improve patient-specific therapy. For the practical application of this scenario, a data protection-compliant integration of the technical components into the existing IT infrastructure is necessary. For these topics, the scientists at the hospital pharmacy are working closely with those at the Institute for Applied Medical Informatics at the UKE.
Integration into Closed Loop Management
Finally, the process is to be connected to the digital medication process of the UKE. The hospital pharmacy of the UKE is a leader in the field of closed loop medication management, which has been introduced throughout the UKE in all disciplines. Physicians' orders are recorded in the electronic prescribing software and transferred to the hospital pharmacy. There, the corresponding drugs are individually packaged and labelled for the patients by computer control. On the ward, the nurse administers the drug after checking the patient's wristband. This ensures maximum patient safety in the medication process.
3D printing of a drug in fast motion
The time-lapse video illustrates the layer-by-layer construction of a tablet from a powder mixture. The printing is carried out by means of a 3D printer specially designed for the production of drugs in the hospital pharmacy of the UKE.
3D printing in pharmaceutics
3D printing of drugs is an innovative manufacturing method that is characterised by a high degree of digitalisation and automation and enables patient-specific care. For example, drugs with a narrow therapeutic index can be individually adapted to a patient and manufactured. This is particularly useful for paediatric applications. Using 3D printing, drugs can be produced that correspond to the quantities and dosages required by children. In addition, the colour, shape and taste could also be adapted - important prerequisites for ensuring that children also accept the recommended treatment and also take their medication. In addition to individual dosages, the technology also offers the possibility of manufacturing tablets that contain several active ingredients - so-called polypills.
Despite the many advantages of 3D printing, it has not yet become established in healthcare. By 2022, there is only one printed drug that has received market approval in the USA. In Europe, there is currently no 3D printed drug ready for the market. This is mainly due to the complicated regulatory processes. In addition, the integration of 3D printing into healthcare is currently also failing due to the requirements of a digital environment. The UKE is considered a pioneer in the digitalisation of clinical processes, has recognised the potential of 3D printing in pharmacy and wants to prove in the EU-funded research project how the integration of 3D printing into the digital clinical environment can succeed.