Experimental Orthopaedic Trauma Surgery
Research Chair:
Prof. Johannes Keller, MD, PhD
Mail:
j.keller@uke.de
Administrative Office:
Ms. Nele Herz
Mail:
n.herz@uke.de
Phone:
Fax: +49 (0) 40 7410 - 40085
-
Overview
Our experimental orthopaedic trauma surgery reasearch lab at the UKE investigates clinically relevant questions in the field of orthopaedic trauma surgery and musculoskeletal medicine through translational research approaches. Through radiological, histological, and molecular investigation of clinical specimens as well as the use of state-of-the-art experimental techniques, we work on traumatological research projects with the overall goal of developing new and innovative therapeutic approaches for affected patients.
One of our main focuses is the identification and functional understanding of local and systemic mediators that are essential for bone regeneration and that can be used pharmacologically to treat impaired bone healing (non-union). Similarly, we are investigating cellular and molecular signaling mechanisms that regulate bone cell function and are thus useful for the treatment of skeletal diseases such as osteoporosis or ectopic bone formation. In cooperation with other university hospitals, we are also investigating the post-traumatic inflammatory response and sepsis, which can lead to complications in severely injured patients due to subsequent organ failure. Biomarkers in post-traumatic joint degeneration, implant-associated infections, heterotopic ossifications, and the application of 3D printing in trauma surgery represent further scientific projects that our team is working on to improve currently available therapeutic options.
In addition to a large number of national and international collaborations, we work closely with the Department of Orthopedics and the Institute of Osteology and Biomechanics (IOBM) at the UKE. This allows a targeted focus of the various core competencies, enabling musculoskeletal research at the highest level.
We would be pleased if you could get an overview of the contents of our translational research activities and our team on the following pages.
-
Team
Research Chair
 Prof. Johannes Keller, MD, PhDJohannes Keller
Prof. Johannes Keller, MD, PhDJohannes Keller- Head of section
Location
Campus Forschung N27 , 1st FloorVice Chair
 Dr. rer. nat.Anke BaranowskyLocation
Dr. rer. nat.Anke BaranowskyLocation
Campus Forschung N27 , 1st FloorMildred-Scheel Junior Scientist
 Location
Location
Campus Forschung N27 , 1st FloorClinician Scientist
 Dr. med.Tobias Malte BallhauseLocation
Dr. med.Tobias Malte BallhauseLocation
Campus Forschung N27 , 1st FloorMedical Resident
 Location
Location
Campus Forschung N27 , 1st FloorPhD-Students
 Location
Location
Campus Forschung N27 , 1st Floor Location
Location
Campus Forschung N27 , 1st Floor Dr. med.Weixin Xie
Dr. med.Weixin Xie- PhD student
Location
Campus Forschung N27 , 1st FloorMD-Students (at least 1-year fulltime in the lab)
 Lilly-Charlotte Albertsen
Lilly-Charlotte Albertsen- MD student
Location
Campus Forschung N27 , 1st Floor Location
Location
Campus Forschung N27 , 1st Floor Location
Location
Campus Forschung N27 , 1st Floor Location
Location
Campus Forschung N27 , 1st Floor Location
Location
Campus Forschung N27 , 1st Floor Location
Location
Campus Forschung N27 , 1st Floor Judith Luisa Kokot
Judith Luisa Kokot- MD student
Location
Campus Forschung N27 , 1st Floor Location
Location
Campus Forschung N27 , 1st Floor Location
Location
Campus Forschung N27 , 1st FloorTechnical Assistants
 Cordula Erdmann
Cordula Erdmann- Technical employee
Location
Campus Forschung N27 , 1st Floor Mayla Rickert
Mayla Rickert- Technical employee
Location
Campus Forschung N27 , 1st FloorAdministrative Office
Location
Main Building O10 , 2nd Floor -
Experimental-Translational Research
Cellular and molecular characterization of fracture healing
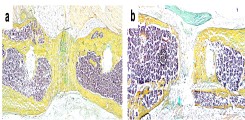
Despite high-quality and safe treatment of bone injury, fractures of long bones do not heal in 10-15% of cases and a so-called non-union develops. This clinical disorder is associated with severe and prolonged pain, multiple revision surgeries, immobility, loss of work and high treatment costs, exposing patients and their families to a high level of suffering. Through our research we want to better understand the pathophysiology of non-unions in order to develop new diagnostic and therapeutic concepts for affected patients.
Fig.: Histological image (Movat pentachrome staining) of a healing (a) and a non-healing (b) long-bone fracture. Mineralized bone appears yellow.
Cellular and molecular basis of osteoporosis
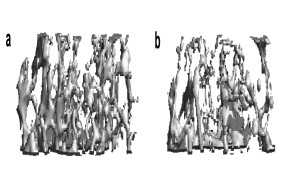
Bone loss (osteoporosis) is the most common bone disease in humans. Although the disease is asymptomatic for a long time, in its advanced stages it results in reduced bone mass and quality and a greatly increased risk of fractures. This disease is triggered by an imbalance in the activity of bone cells, the bone-forming osteoblasts and the bone-resorbing osteoclasts. In our research, we investigate the regulation of these cells with the aim of identifying novel factors that counteract bone resorption and promote the formation of new bone.
Fig.: Radiological imaging (µCT) of altered bone structure in systemic bone loss disorders. (a) Normal trabecular bone architecture. (b) Rarified and thinned trabecular structure in post-menopausal osteoporosis.
Molecular biology analysis of post-traumatic osteoarthritis
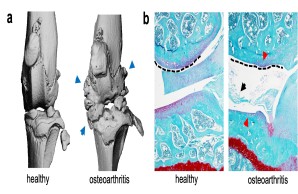
Joint injury is a common cause of post-traumatic joint degeneration (osteoarthritis). Apart from symptomatic treatments, currently only artificial joint replacement is available for the treatment of advanced osteoarthritis. In our research group, we are therefore investigating the pathophysiology of post-traumatic osteoarthritis using state-of-the-art experimental methods in order to develop new treatment strategies for affected patients in the long term.
Fig.: Radiological (µCT; a) and histological (b) imaging of joint changes in post-traumatic osteoarthritis. The classic signs of osteoarthritis appear with osteophytes (blue arrows), meniscal degeneration (black arrow), joint deformation (dashed line), and loss of articular cartilage (red arrows).
Cellular communication mechanisms in osteoimmunology
The interaction of bone and immune cells plays a major role in many diseases. This interaction is not only important in normal or pathological bone remodeling (i.e., osteoporosis), but also controls degenerative (e.g., osteoarthritis) and regenerative processes (e.g., fracture healing). In our work, we therefore characterize the influence of specific immune cells on bone remodeling, joint degeneration and bone healing, and investigate to what extent immunomodulatory substances are suitable for the treatment of skeletal diseases.
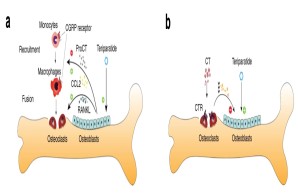
Fig.: Interaction of bone cells with immune cells after osteoanabolic teriparatide therapy. (a) Teriparatide induces procalcitonin (ProCT) expression in osteoblasts, which impairs the recruitment of osteoclast precursors (i.e., monocytes and macrophages) to the bone surface, thereby inhibiting bone resorption. (b) Circulating calcitonin (CT) impairs the osteoanabolic effect of iPTH based on tonic inhibition of bone formation, presumably via osteoclasts. Modified from Baranowsky et al, Bone Research 2022.
Inflammation and regeneration after spinal trauma
Spinal trauma is still a serious clinical picture with severe consequences for patients today. During the secondary phase after spinal cord injury, the inflammatory response in particular leads to progressive cell death and thus to progressive neuronal cell death and consecutive deterioration of motor function. Using state-of-the-art experimental methods, we are investigating the effects of specific immunomodulatory substances on the secondary phase with the aim to improve spinal regeneration and clinical outcomes.
Regulation of the post-traumatic inflammatory response and sepsis
After severe trauma, an excessive immune response often takes place in the organism, which can restrict the function of important organs. If an additional bacterial infection occurs, a life-threatening sepsis with multi-organ failure can develop within a short time due to immune dysregulation. In our research group, we are therefore investigating which factors are decisively involved in the immunological processes of sepsis and to what extent these can be pharmacologically influenced to improve the clinical outcome.
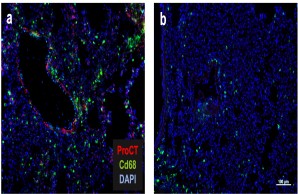
Fig.: The role of procalcitonin in post-traumatic sepsis. The sepsis biomarker procalcitonin (ProCT) is not only used to diagnose bacterial sepsis, but also functions as a pathophysiological mediator in this disease. ProCT enhances the generalized inflammatory response in sepsis, leading to tissue injury, shown here in immunofluorescence of macrophages (Cd68) in lung preparations (a). If procalcitonin is blocked during sepsis, the inflammatory response and tissue damage are much less pronounced (b). Modified from Baranowsky et al, Critical Care Medicine 2021.
Infections of bone and implants
Bacterial infections of bone and osteosynthetic implants are rare but serious complications in trauma surgery. Our research group therefore investigates how bacteria and their biofilm interact with bone and cartilage cells. From these investigations, for example, novel biomarkers can be derived that can be used to diagnose peri-implant infections. In addition, these investigations can contribute to improving the effectiveness of existing therapeutic approaches. The project is being carried out in close cooperation with the Orthopedics, the IOBM and the Rohde Lab (Department of Medical Microbiology, Virology and Hygiene, UKE).
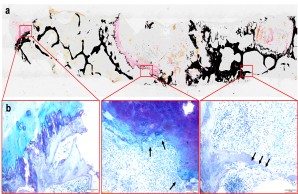
Fig.: Nondecalcified histologic workup of bacterial inflammation of the intervertebral disc with osseous infiltration of the vertebral body. (a) Von Kossa/van Gieson overview staining. (b) Detailed images in toluidine blue staining. Arrows show highly activated osteoclasts responsible for vertebral bone destruction. Joint project with Orthopedics (PD Dr. Dr. Tim Rolvien), IOBM (Prof. Dr. Michael Amling) and Microbiology (Prof. Dr. Holger Rohde).
-
Clinical-Translational Research
Biomarkers of traumatic knee joint disorders
Internal knee injuries are among the most common musculoskeletal injuries, often affecting young and physically active people. These include, for example, ruptures of the anterior cruciate ligament and meniscus lesions, which are treated at the highest level in our Department of Trauma Surgery and Orthopedics. Despite state-of-the-art therapeutic procedures, however, it remains unclear why individual patients show an unsatisfactory healing process. Therefore, we are investigating specific biomarkers and molecular mediators in the knee joint, which should provide prognostic information on clinical outcome and allow individualized treatment of patients with internal knee injuries in the long term. The clinical part of the study is led by PD Dr. Matthias Krause and Prof. Dr. Karl-Heinz Frosch .
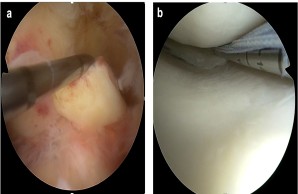
Fig.: Internal knee trauma as a typical example of common musculoskeletal injuries. (a) Fresh tear of the anterior cruciate ligament (ACL). Here, the ACL is pulled forward with a hook probe during arthroscopic exploration. (b) Arthroscopic suture of a torn meniscus in the posteromedial joint corner.
Molecular footprint of non-unions
Failure of bone healing after fracture remains a major clinical challenge in trauma surgery, affecting elderly and pre-diseased patients as well as young and working patients. Accordingly, improving bone regeneration is of great clinical importance. Based on the results of our basic research in this area, we are clinically investigating the role of different candidate proteins in impaired fracture healing. The overall goal of this study is to be able to apply our findings to improve bone regeneration in all patients with impaired bone healing. The clinical part of the study is led by Dr. Holger Kleinertz and Dr. Carsten Schlickewei .
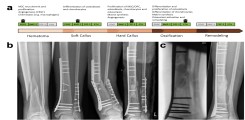
Fig.: Fracture healing. (a) Course of regular fracture healing and growth hormones involved. (b) Case report with delayed fracture healing and peri-implant fracture. (c) Healing after multistage therapy using Masquelet technique and bone graft with autologous cancellous bone. Modified after Schlickewei, Kleinertz et al., IJMS 2019.
Structural and molecular analysis of heterotopic ossification
Ectopic bone formation (heterotopic ossification) following trauma leads to lasting functional limitations, particularly in the elbow and hip joints, beginning with decreased mobility, compression of nerves and vessels, and multiple revision surgeries. In our work, we are investigating structural, cellular, and molecular mechanisms that contribute to the onset and progression of this disease. Dr. Milad Fal and PD Dr. Konrad Mader are leading the clinical portion of the study.
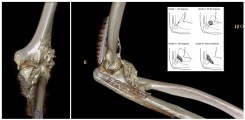
Fig.: 3D-rendered CT of the left elbow in a 25-year-old male with complex trauma to the elbow. Post-traumatically, a massive heterotopic ossification (HO) is formed ventrally and radially in the soft tissues. In the insert, a schematic drawing explaining the classification of HO at the elbow according to Ilahi.
3D-printing of bones and joints for clinical, research, and teaching applications
3D-printing is becoming increasingly important in modern university medicine. Especially in trauma surgery, 3D-printing is experiencing a technological boom in the diagnosis, planning, and treatment of fractures and complex reconstructive procedures. With our research, we are exploring different application areas of 3D-printing in traumatology to enable the implementation of this technology in individualized treatment concepts in the long term. Our 3D-printing projects are supervised by Dr. Tobias Dust and PD Dr. Konrad Mader .
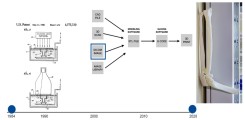
Fig.: Evolution of medical 3D-printing: on the left, Chuck Hall's original patent drawing from 1986; in the center, the process from image dataset to 3D-print; and on the right, a 3D-print of a CT dataset of a patient with complex (transolecranon) dislocation fracture of the right elbow.
-
Selected Publications
Jiang, S., Xie, W., Knapstein, P. R., Donat, A., Albertsen, L.-C., Sevecke, J., Erdmann, C., Appelt, J., Fuchs, M., Hildebrandt, A., Maleitzke, T., Frosch, K.-H., Baranowsky, A., & Keller, J. (2024). Transcript-dependent effects of the CALCA gene on the progression of post-traumatic osteoarthritis in mice. Communications Biology, 7(1), 223. https://doi.org/10.1038/s42003-024-05889-0
Xie, W., Jiang, S., Donat, A., Knapstein, P. R., Albertsen, L.-C., Kokot, J. L., Erdmann, C., Rolvien, T., Frosch, K.-H., Baranowsky, A., & Keller, J. (2024). Tranexamic Acid Attenuates the Progression of Posttraumatic Osteoarthritis in Mice. The American Journal of Sports Medicine, 52(3), 766–778. https://doi.org/10.1177/03635465231220855
Jahn, D., Knapstein, P. R., Otto, E., Köhli, P., Sevecke, J., Graef, F., Graffmann, C., Fuchs, M., Jiang, S., Rickert, M., Erdmann, C., Appelt, J., Revend, L., Küttner, Q., Witte, J., Rahmani, A., Duda, G., Xie, W., Donat, A., … Keller, J. (2024). Increased β2-adrenergic signaling promotes fracture healing through callus neovascularization in mice. Science Translational Medicine, 16(743), eadk9129. https://doi.org/10.1126/scitranslmed.adk9129
Messerer, D. A. C., Datzmann, T., Baranowsky, A., Peschel, L., Hoffmann, A., Gröger, M., Amling, M., Wepler, M., Nussbaum, B. L., Jiang, S., Knapstein, P., Donat, A., Calzia, E., Radermacher, P., & Keller, J. (2022). Systemic calcitonin gene-related peptide receptor antagonism decreases survival in a porcine model of polymicrobial sepsis. BRIT J ANAESTH, 128(5), 864-873. https://doi.org/10.1016/j.bja.2021.11.042
Grewe, J. M., Knapstein, P-R., Donat, A., Jiang, S., Smit, D. J., Xie, W., & Keller, J. (2022). The role of sphingosine-1-phosphate in bone remodeling and osteoporosis. BONE RES, 10(1), [34].
https://doi.org/10.1038/s41413-022-00205-0Baranowsky, A., Jahn, D., Jiang, S., Yorgan, T., Ludewig, P., Appelt, J., Albrecht, K. K., Otto, E., Knapstein, P., Donat, A., Winneberger, J., Rosenthal, L., Köhli, P., Erdmann, C., Fuchs, M., Frosch, K-H., Tsitsilonis, S., Amling, M., Schinke, T., & Keller, J. (2022). Procalcitonin is expressed in osteoblasts and limits bone resorption through inhibition of macrophage migration during intermittent PTH treatment. BONE RES, 10(1), [9].
https://doi.org/10.1038/s41413-021-00172-yMaleitzke, T., Hildebrandt, A., Weber, J., Dietrich, T., Appelt, J., Jahn, D., Zocholl, D., Baranowsky, A., Duda, G. N., Tsitsilonis, S., & Keller, J. (2021). Proinflammatory and bone protective role of calcitonin gene-related peptide alpha in collagen antibody-induced arthritis. RHEUMATOLOGY, 60(4), 1996-2009.
https://doi.org/10.1093/rheumatology/keaa711Baranowsky, A., Appelt, J., Kleber, C., Lange, T., Ludewig, P., Jahn, D., Pandey, P., Keller, D., Rose, T., Schetler, D., Braumüller, S., Huber-Lang, M., Tsitsilonis, S., Yorgan, T. A., Frosch, K-H., Amling, M., Schinke, T., & Keller, J. (2021). Procalcitonin Exerts a Mediator Role in Septic Shock Through the Calcitonin Gene-Related Peptide Receptor. CRIT CARE MED, 49(1), e41-e52.
https://doi.org/10.1097/CCM.0000000000004731Appelt, J., Baranowsky, A., Jahn, D., Yorgan, T. A., Köhli, P., Otto, E., Farahani, S. K., Graef, F., Fuchs, M., Herrera, A., Amling, M., Schinke, T., Frosch, K-H., Duda, G. N., Tsitsilonis, S., & Keller, J. (2020). The neuropeptide calcitonin gene-related peptide alpha is essential for bone healing. EBIOMEDICINE, 59, 102970.
https://doi.org/10.1016/j.ebiom.2020.102970Keller, J., Catala-Lehnen, P., Hübner, A. K., Jeschke, A., Heckt, T., Lueth, A., Krause, M., Koehne, T., Albers, J., Schulze, J., Schilling, S., Haberland, M., Denninger, H., Neven, M., Hermans-Borgmeyer, I., Streichert, T., Breer, S., Barvencik, F., Levkau, B., ... Amling, M. (2014). Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. NAT COMMUN, 5, 5215.
https://doi.org/10.1038/ncomms6215Albers, J.*, Keller, J.*, Baranowsky, A.*, Beil, F. T., Catala-Lehnen, P., Schulze, J., Amling, M., & Schinke, T. (2013). Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J CELL BIOL, 200(4), 537-49. *equal contribution.
https://doi.org/10.1083/jcb.201207142 -
Funding
Deutsche Forschungsgemeinschaft
Else-Kröner-Fresenius-Stiftung
Clinician Scientist Programm UKE
Mildred-Scheel Nachwuchszentrum
Berlin Institute of Health
Charité 3R
Jung-Stiftung
We would like to express our sincere thanks to all funding institutions for their support of our work.
-
Doctoral Theses and Student Research Projects
Experimental orthopaedic trauma surgery offers challenging doctoral and undergraduate theses to interested students. Enthusiasm for trauma surgery, utmost commitment, creative and problem-oriented thinking, and the ability to work independently are essential prerequisites. First and co-authorship of publications and contributions to national and international congresses are sought, whenever possible, for all MD and PhD students.
We ask for your understanding that only a limited selection of suitable candidates can be considered in order to ensure high quality supervision.
If you are interested, we look forward to hearing from you @: j.keller@uke.de.